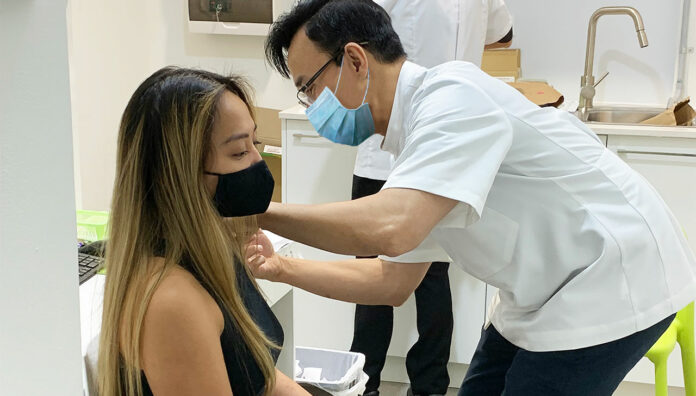
The Therapeutic Goods Administration has provisionally approved the Pfizer COVID-19 vaccine as a booster dose for all Australians aged over 18, Federal Minister for Health Greg Hunt announced today.
In a press conference this morning, Mr Hunt called the booster program an ‘important step’.
‘It means that Australia will be one of the most highly vaccinated societies in the world, one of the most recently vaccinated countries in the world, and one of the first to receive a whole-of -population booster program,’ he said.
The ‘universal’ booster dose is available to patients 6 months after the completion of their primary COVID-19 vaccination course, whether they received the AstraZeneca, Moderna or Pfizer vaccines.
The Technical Advisory Group on Immunisation (ATAGI) is expected to provide final advice on the roll out of Pfizer booster doses shortly. Once ATAGI has provided its recommendation, the general population booster program is expected to roll out by 8 November at the latest.
Several groups will be prioritised, however, including people in aged care and disability care settings.
In Victoria, which has ‘the most significant current outbreak, we’ll be looking to commence their aged care and disability support booster for those that are 6 months plus, since they’ve had their second dose, in the imminent future,’ Minister Hunt said.
Moderna is expected to apply for registration of booster doses for its vaccine shortly, but the Pharmacy Programs Administrator (PPA) advised this morning that pharmacies currently ‘onboarded’ to deliver Spikevax will be able to order and administer the Pfizer vaccine.
‘We will be making Pfizer available [in] pharmacies, because at this stage there hasn’t been an application and approval for Moderna as a booster, [and] we want to make sure that [it] is available, Mr Hunt said.
PSA National President, Associate Professor Chris Freeman, welcomed the news that pharmacists will be included in the Pfizer booster dose rollout, and commended the Federal Government’s leadership.
‘Today is a day of significant progress in Australia’s COVID-19 response, as over 3,000 pharmacies who have already administered more than 1.5 million vaccinations, now have the opportunity to order and administer the Pfizer vaccine,’ he said.
‘This will enable eligible pharmacist immunisers to further protect their communities following the completion of the primary double-dose course.’
Pharmacists to drive accessibility
PSA’s Communities of Specialty Interest lead for Contemporary Community Pharmacy Practice, Dr Fei Sim FPS, said granting pharmacists access to the Pfzier vaccine demonstrated a commitment to achieving vaccination targets.
‘Community pharmacies are so widespread, we are the most accessible healthcare professionals,’ she told Australian Pharmacist.
‘Having a [booster] dose rollout in community pharmacies [means] enabling timely and equitable access to third dose vaccines to all Australians.’
This is particularly important in regional, rural, and remote areas, where community pharmacies are often the only available healthcare contact.
Dr Sim also anticipates the move will dispel vaccine hesitancy centered around rare incidents of thrombosis with thrombocytopenia syndrome associated with the AstraZeneca vaccine.
‘When it comes to preference, people seem to have the least hesitancy towards the Pfizer vaccine,’ she said.
‘Having the vaccine available in community pharmacies will help to enable that access, and [allow] the community to have autonomy around the vaccine they receive.’
Once the Moderna booster application is approved, the vaccine choice in community pharmacy will widen further.
PSA has strongly advocated for pharmacist immunisers to have access to all approved COVID-19 vaccines – simplifying vaccination options for patients and giving Australians greater choice.
Preparing for Pfizer
While there have been concerns about the ability of community pharmacists to administer the Pfizer vaccine due to storage issues, Dr Sim said pharmacists have risen to the occasion throughout the pandemic.
‘Just look at the rollout of AstraZeneca and Moderna vaccines in community pharmacies,’ she said.
‘Pharmacists can [manage] cold chain, and community pharmacies are well set up to manage COVID-19 vaccinations [while] meeting cold chain requirements, so the same will apply to the Pfizer vaccine.’
To prepare, the PPA said pharmacies wanting to administer the Pfizer vaccine must ensure staff complete the specific Pfizer vaccine training module as soon as possible.
Pharmacists can order Pfizer vaccine stock in the COVID-19 Vaccine Ordering System (CVAS) in the coming week. Following ATAGI’s recommendation, pharmacies that are administering Spikevax will receive a CVAS invitation.
Once a Site Readiness Checklist and Pfizer Declaration Form have been completed, pharmacists will be able to complete their order.
As the booster program rolls out, PSA will continue to support pharmacists through CPD, practice-support and advocacy.
Rapid antigen testing needed in pharmacies
Meanwhile, PSA is calling on governments to protect the frontline healthcare workforce, and to ensure pharmacies remain open, by providing rapid antigen tests (RAT) for pharmacists and pharmacy staff.
‘With jurisdictions reopening, it’s likely we will continue to see a large number of pharmacies identified as exposure sites,’ A/Prof Freeman said.
‘Staff will still have to isolate and stores may have to temporarily close until PCR results are returned and deep cleaning has concluded [but] RAT expedites this process, returning results within 15 minutes.’
While professional football teams, schools, abattoirs and various other industries are already using RAT, pharmacists and pharmacy staff continue putting themselves at risk on a daily basis to serve the community.
‘It’s time to protect our frontline health workers by providing rapid antigen tests for pharmacists,’ A/Prof Freeman said.



 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more