Community pharmacists could more frequently give patients a choice between the two emergency contraception drugs available in Australia.
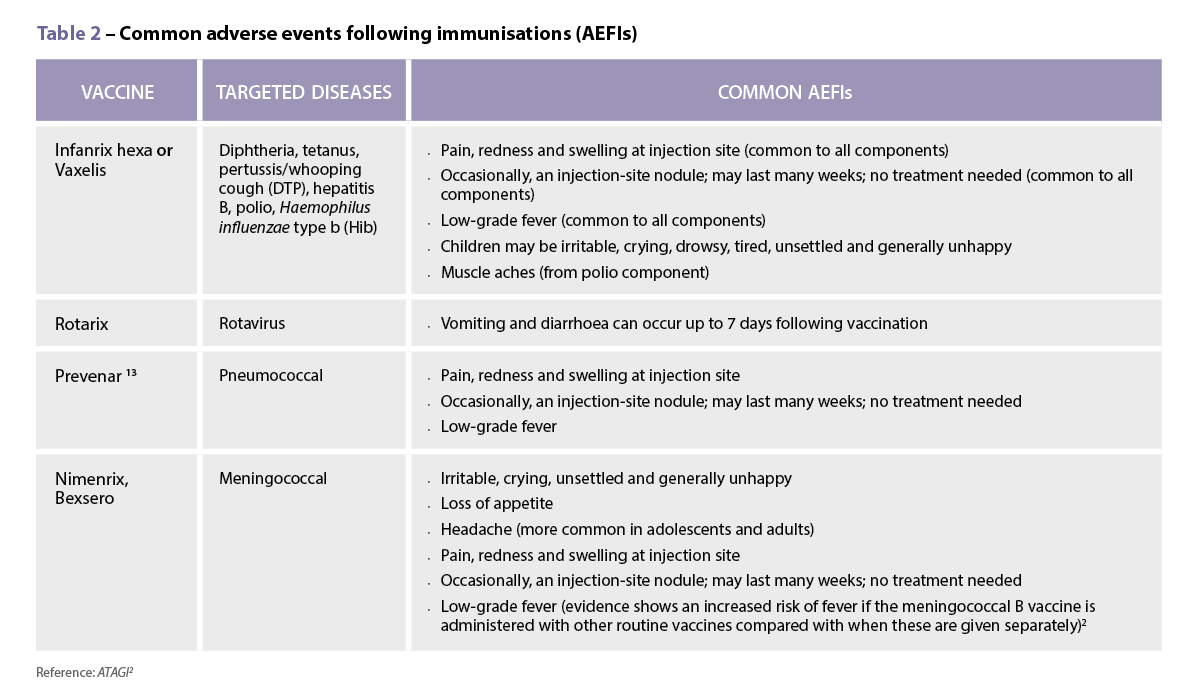
The mystery shopper exercise conducted by University of Sydney researchers found that emergency contraception drug levonorgestrel (LNG) was provided 74% of the time, while ulipristal (UPA) was provided on 26% of occasions.
‘The 26% figure is not necessarily concerning, with the exception of the few cases where LNG was supplied in the 72-120 hour window,’ said University of Sydney Pharmacy School researcher and PhD candidate Jack Collins.
‘What was interesting to find from the study was that pharmacists were not offering consumers the choice between the two agents, but instead making the decision on their behalf,’ he said.
The study, published in the Contraception Journal, involved 20 pharmacy students mystery-shopping at 10 community pharmacies in metropolitan Sydney and requesting ‘the morning-after pill’.
Mr Collins said the study highlighted three key issues for pharmacists when it came to supplying emergency contraception (EC).
Firstly, pharmacists needed to ensure that their knowledge of current products, legislation, and guidelines was up to date.
‘Pharmacist knowledge of, and comfort in supplying, ulipristal acetate (sold under the brand name EllaOne) has room for improvement,’ Mr Collins said.
‘Many pharmacists did not feel they possessed adequate knowledge to supply UPA, and/or were satisfied that LNG would suffice for the pseudopatients.’
There was also a need to look at the pharmacist’s role in consumer self-agency, he said.
‘Few of our pseudopatients were offered a choice between LNG and UPA, and while pharmacists did offer explanation as to why they selected one drug over the other, such as cost, it is important that pharmacists try to shift towards a shared decision-making model where pharmacists discuss the pros and cons of the options and come to a decision together with the consumer,’ he said.
‘A final issue was some misunderstandings around the regulation of supply of EC. NSW law does not prohibit supply to anyone under 16 years of age or to a third party (such as a male partner),’ Mr Collins said.
According to Professional Practice Standards criterion (1.1.3) a pharmacist is required to respond to patients, authorised representatives and other healthcare professionals in a timely manner to ensure that the health needs of patients and the community are met in a consistent manner.
Refusing to supply because the patient is not present in the pharmacy, without exploring other options to meet their needs, compromises the patient’s ability to access treatment in a timely manner.
Pharmacists can access PSA’s EC guidance document or undertake the society’s online CPD here.
Here are Mr Collins’ top six things Australian pharmacists should know about UPA:
- There are now two options for EC: LNG and UPA.
- When first launched, UPA was licensed as being non-inferior to LNG. More contemporary research suggests that it is more efficacious than LNG at inhibiting ovulation with a comparable adverse effect profile.
- Both LNG and UPA are licensed for use up to 72 hours post-intercourse. UPA is also licensed up to 120 hours post-intercourse.
- UPA exerts its inhibitory action by modulating the progesterone receptor where it demonstrates both agonist and antagonist activity.
- Oral contraceptive pills should not be commenced or re-commenced for 5 days following administration of UPA.
- Neither UPA or LNG is effective at preventing pregnancy if ovulation has already occurred and the two should not be used together as EC.


 Professor Anthony Lawler, Australian Government Chief Medical Officer,
Professor Anthony Lawler, Australian Government Chief Medical Officer, 
 This CPD activity is supported by an unrestricted education grant by Reckitt.[/caption]
This CPD activity is supported by an unrestricted education grant by Reckitt.[/caption]




 Jess Hadley, community pharmacist and Professional Officer at PDL[/caption]
Jess Hadley, community pharmacist and Professional Officer at PDL[/caption]
 Peter Guthrey, Senior Pharmacist – Strategic Policy at PSA[/caption]
Peter Guthrey, Senior Pharmacist – Strategic Policy at PSA[/caption]








