Findings from a recent Cochrane review.1
Background
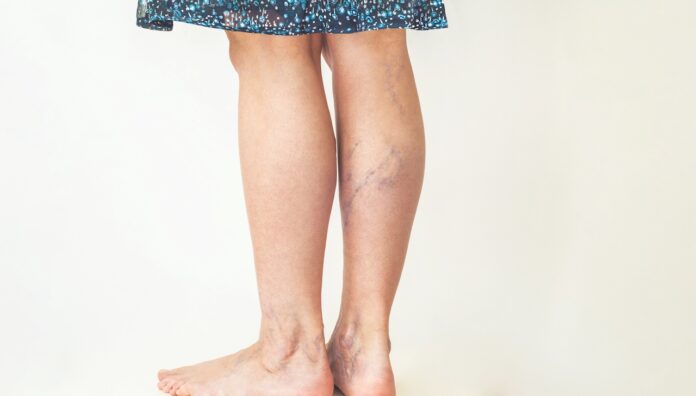
Venous thromboembolism (VTE) is the third most common cardiovascular disease in the world after stroke and myocardial infarction.2 VTE is the formation of a thrombus (blood clot) in the deep veins such as the legs (deep venous thrombosis or DVT) or in the pulmonary circulation (pulmonary embolism or PE), or both.1,3 Every year, 30,000 people in Australia develop VTE, at a cost of $1.72 billion to the Australian health system.4
Anticoagulants are generally considered the standard treatment for DVT. These medicines reduce the formation of clots and prevent further VTE. Other options for treatment of DVT include thrombolysis, mechanical thrombectomy, angioplasty and stenting. Antiplatelet medicines, such as aspirin, are routinely used in clinical practice to treat various cardiovascular conditions. The routine use of aspirin has been established in cases of arterial thrombosis with a cardiac, cerebrovascular or peripheral origin.
Clinical evidence suggests that antiplatelet medicines can prevent the onset and spread of venous thrombus, minimising the adverse events of PE and DVT. However, overseas guidelines regarding antithrombotic therapy for VTE disease do not recommend antiplatelet medicines in people with DVT.5
This summary will present the benefits of antiplatelet medicines in addition to current best medical treatment (BMP), which includes anticoagulation, compression stockings and clinical care (such as physical exercises, skin hydration, etc), compared to current BMP (with or without placebo) for the treatment of DVT.
Characteristics of the studies
The studies included in the review were randomised controlled trials (RCTs) examining antiplatelet medicines compared to BMP following initial standard anticoagulation treatment for DVT. The review included all RCTs of antiplatelet therapy plus current BMP (including anticoagulants, compression stockings and clinical care such as physical exercises, skin hydration, etc) versus BMP with or without placebo for the treatment of DVT.
Quality of the study
The quality of the evidence for the studies included were rated as very low to moderate due to randomisation, indirectness, imprecision, publication bias and study limitation.
Results
- The following databases were searched: Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS-Web), Cochrane Central Register of Controlled Trials (CENTRAL; 2021 Issue 11) via the Cochrane Register of Studies Online (CRSO), MEDLINE (Ovid MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE, Embase Ovid, CINAHL Ebsco and AMED Ovid. Other trial registries searched were the World Health Organization International Clinical Trials Registry Platform (who.int/trial search) and ClinicalTrials.gov.
- The primary outcome measures were recurrent VTE, major bleeding and incidence of PE.
- The secondary outcome measures were mortality: all cause and VTE-related, post-thrombotic syndrome (PTS), adverse events, quality of life and duration of hospitalisations.
- The review included six studies with 1,625 eligible participants, one study followed up after 37.2 months. For acute DVT, there were no studies included comparing antiplatelet medicines plus BMP versus BMP plus placebo.
- In acute DVT, antiplatelet medicines plus BMP versus BMP alone were included in one study with a total of 500 participants, which reported on four outcomes for 6 months of follow-up. There were no deaths and no cases of major bleeding reported. The participants who received antiplatelet agents showed a lower risk of PTS (RR 0.74, 95% CI 0.61–0.91; based on very low-certainty evidence). The control group presented a lower risk of adverse events compared to the intervention group (RR 2.88, 95% CI 1.06–7.80; based on very low-certainty evidence).
- In chronic DVT, antiplatelet medicines plus BMP versus BMP alone were included in one study (224 participants). Four relevant outcomes were reported, three (major bleeding, mortality and adverse events) showing no events during the 3 years of follow-up.
- In chronic DVT, antiplatelet medicines plus BMP versus BMP plus placebo were reported in four studies (901 participants). Meta-analysis of this data showed a lower risk of recurrent VTE for the antiplatelet medicines group (RR 0.65, 95%, CI 0.43–0.96; NNTB = 14; based on low-certainty evidence). For major bleeding, there was no clear difference between placebo and intervention groups until 37.2 months of follow-up (RR 0.98, 95% CI 0.29–3.34; based on moderate-certainty evidence).
- In PE fatal/non-fatal outcome, there was no clear difference with the use of antiplatelet medicines (RR 0.52, 95% CI 0.23–1.14; one study, based on moderate- certainty evidence). For all-cause mortality, the overall effect of antiplatelet medicines did not differ from the placebo group (RR 0.48, 95% CI 0.21–1.06; based on moderate- certainty evidence). Adverse events outcome showed no difference (RR 1.57, 95% CI 0.34–7.19 on moderate-certainty evidence).
Conclusion
For acute and chronic DVT, based on the limited available evidence, no conclusion can be drawn for using antiplatelet medicines in addition to BMP after standard initial treatment with anticoagulants, when compared to BMP alone. There is no clear difference in adverse effects, major bleeding or PE with the use of antiplatelet medicines in addition to BMP when compared to BMP plus placebo.
Implications for practice and research
The included studies in this review were relatively small and varied in quality. Further research should address larger trials with robust methodologies and include people with acute and chronic DVT with long periods of follow-up. It is also recommended to include information about important outcome measures such as DVT, PE, major bleeds and quality of life.
References
- Flumignan CDQ, Nakano LCU, Baptista-Silva JCC, et al. Antiplatelet agents for the treatment of deep venous thrombosis. Cochrane Database of Systematic Reviews 2022, Issue 7.
- Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol 2012;25(3):235–42.
- Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE study cohort. Ame J Med 2013;126(9):832.e13–21.
- Australian Commission on Safety and Quality in Healthcare. Blood clot prevention care to save lives and reduce $1.7b burden on health system. (2018).
- National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing.
ASSOCIATE PROFESSOR HANAN KHALIL BPharm, MPharm, PhD, AACPA is the Lead of Health Services Administration at Latrobe University. She is also Co-Editor in Chief of the JBI Evidence Implementation.


 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more