Coming soon: Greater online access for pharmacists to pathology and diagnostic imaging reports, a crucial boost for patient safety.
Pathology tests and diagnostic imaging are a critical part of health care. Test results are important for diagnosis, to track disease progression and to assess the impact of treatments, especially medicines, on the body.
Pharmacists are medicine experts, with rapidly expanding expertise in identifying when medicines are unsafe, inappropriate or ineffective. Crucial to determining medicines safety is access to up-to-date pathology and diagnostic imaging information at the time patient care is provided.
Hospital pharmacists have had full access to hospital pathology systems for years. Pathology results are routinely reviewed as part of the care provided by pharmacists working in hospital teams, including outpatient services. But pharmacists in other settings often haven’t necessarily had ready access to test results, instead having to rely on patient recall or requests to medical doctors for critical information such as liver function, kidney function and electrolyte levels.
This long-term norm, where most pharmacists do not have timely access to essential pathology report information, prevents pharmacists from making sound judgement calls for patient health. So, are things changing? Or will we forever be ‘dispensing in the dark’?
Change is coming
In line with the recommendations of the Strengthening Medicare Taskforce,1 public and private pathology and diagnostic imaging providers will soon be required to share their reports to My Health Record (MHR) by default.
This mandatory sharing of health information via MHR upload will become a legal requirement, potentially by the end of 2024.2 The exact timing of the change will be determined by the Government, says a spokesperson from the Department of Health and Aged Care.
‘The establishment of legislative requirements to upload pathology and diagnostic imaging reports is subject to decisions of Government and the Australian Parliament,’ the department spokesperson told AP.
This change will remove barriers to all types of pharmacists viewing pathology results and increase patient safety.
Pharmacists will be better able to determine whether there is any danger to a patient from a particular prescription medicine – e.g. if it can affect potassium concentration and the blood test results show levels which could result in heart complications, among other effects.
With far more light on the subject, all pharmacists will be able to immediately reference pathology results to better tailor their advice to patients regarding certain medicines.
Professional Practice Standards requirements
PSA’s Manager – Practice Support Claire Antrobus says a thorough assessment of patients’ needs is part of the Professional Practice Standards,3 adding this assessment may include reviewing or requesting relevant information such as pathology reports from MHR.
‘If you haven’t gathered the relevant information to establish the person’s needs, it may be difficult to determine if the therapeutic good is safe and therapeutically appropriate for the patient,’ she says.
‘A pharmacist might also use information in a patient’s My Health Record when compiling a best possible medication history for the patient as part of a medication review, or when completing a risk assessment to determine the appropriateness of a medicine for administration to a patient.’
Ms Antrobus says there is useful information available to all pharmacists on how best to use laboratory tests results in clinical pharmacy practice.
‘The Australian Pharmaceutical Formulary and Handbook (APF)4 contains a great chapter on laboratory tests used in clinical practice,’ she says. ‘This chapter outlines some common laboratory tests that are performed, the usual reference ranges, and what a result that is higher or lower than the usual reference range might indicate.’
According to APF, pharmacists should ‘gather sufficient information to assess the safety and appropriateness of a medicine for the patient’, she adds.
Daily use of MHR should be the norm
Dr Shane Jackson, PSA National Board Member and a practising pharmacist in community and consultant pharmacy, says he accesses MHR data daily.
‘We have a large proportion of older people in one of my practices, and we review discharge summaries to make sure issues haven’t been missed.’
He says he recently saw an individual with hypercalcaemia and was able to understand the extent of the issue by accessing the individual’s MHR.
Dr Jackson and his colleagues usually devote 1 day a week to Home Medicines Reviews (HMRs), and they access up-to-date pathology results in MHR and other relevant information for the reviews.
Pathology data, he says, is relevant for all pharmacists, including those working in community pharmacies, in hospitals and as credentialed pharmacists. ‘The frequency with which a pharmacist might be accessing information might be different, and for different reasons,’ he adds, ‘but access nonetheless is relevant across all cohorts.’
There are many reasons for pharmacists to access MHR, Dr Jackson says. A pharmacist might need to know whether a patient is reaching his or her lipid target, or simply the date of their last test.
Discharge summary information about the type or severity of heart failure might be needed so a pharmacist can tailor advice and information, and values indicating the extent of an individual’s renal impairment are needed when providing COVID antivirals, for instance.
‘It’s a case-by-case basis, but given access to extra information, we are no longer dancing in the dark,’ says Dr Jackson.
Future expected pharmacy practice
As a summary of key health information, MHR includes records of immunisations, pathology and diagnostic imaging reports, prescription and dispensing information, and discharge summaries from hospitals.
A spokesperson from the Australian Digital Health Agency estimates that currently only about half of all pathology reports and about 1 in 5 diagnostic imaging reports are being uploaded to MHR, but the coming reform will make it mandatory to upload the reports to ensure healthcare professionals have access to important patient information.
‘The Agency is supporting the Department of Health and Aged Care on a significant policy reform that will see the mandatory sharing of key health information to My Health Record, starting with pathology and diagnostic imaging reports,’ the spokesperson said.
The reform will significantly increase the amount of high-value clinical content uploaded to the system, added the spokesperson, although exceptions will include those instances in which a consumer does not have a MHR or has asked that the report not be uploaded.
Consumers will continue to have the choice of whether or not to have an MHR, as well as which information can be added to their record and how it is managed, including which healthcare provider organisations have access to the record, says the spokesperson for the Department of Health and Aged Care.
 ‘The scope of the types of pathology and diagnostic reports that will be covered by the new legislation is yet to be finalised, however it is expected that the majority of reports will be considered in scope,’ the spokesperson says.
‘The scope of the types of pathology and diagnostic reports that will be covered by the new legislation is yet to be finalised, however it is expected that the majority of reports will be considered in scope,’ the spokesperson says.
New and more focused pathology tests
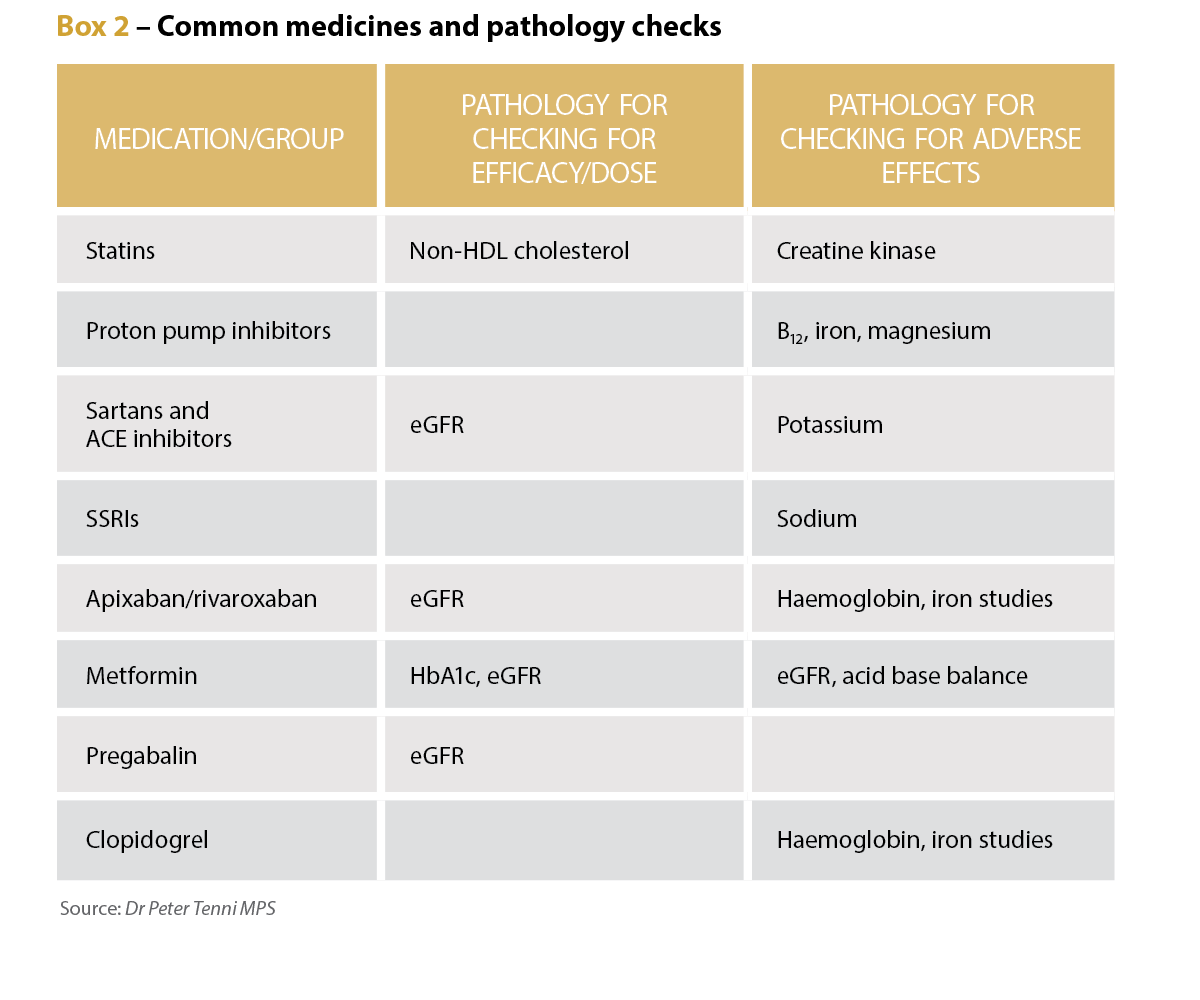
Long-term credentialed pharmacist and educator Dr Peter Tenni MPS says the number and range of pathology tests have increased dramatically over the past 40 years, and there are now more specific and useful tests available for common conditions such as diabetes, heart disease and anaemia.
In the early days, he says, pharmacists’ involvement with pathology results was effectively limited to hospital settings. ‘Since then, as the clinical roles of pharmacists have expanded (such as conducting medication reviews, working in medical clinics and in nursing homes), pharmacists in all areas of practice have had increased access to this important information,’ Dr Tenni says. ‘Pharmacists can add value to the medication use process by having access to appropriate pathology information, and I believe that this is now becoming generally accepted by other health professionals.’
Pathology tests have a variety of uses for pharmacists, he says, including determining a patient’s renal function to confirm the suitability of the dose of renally cleared medicines, and to find any adverse effects of medicines by checking potassium levels in patients taking diuretics, and renal function for patients taking NSAIDS.
The tests can also be used to screen for diabetes, hyperlipidaemia, anaemia and thyroid disorders, Dr Tenni adds. They can also be used to assess the efficacy of medicines, such as checking BNP for patients with heart failure, and iron studies for those on iron supplements, as well as HbA1c for patients with diabetes.
‘Credentialed pharmacists frequently check the pathology parameters to ensure the appropriateness of any medication, to check for any adverse impact of medications, and to check for the effectiveness of medication,’ he says.
In a community setting, however, a pharmacist might not be aware of the details of medical conditions and may decide which pathology is relevant based on the medication regimen, he adds, potentially to check dose appropriateness (such as renal function with pregabalin) and any adverse effects, such as hyponatraemia with SSRIs.

Important tool for patient care
Dr Tenni says if a new dose or new medicine has been prescribed, checking pathology reports for contraindications is reasonable. For instance, a new prescription for spironolactone or a dose increase for an ACE inhibitor or an angiotensin II receptor blocker warrants a pathology check to ensure the patient’s potassium is not elevated.
‘If the patient presents with a potentially medication-related symptom or sign, then it may also be reasonable to check for pathology-related causes or contributors,’ Dr Tenni says, citing signs consistent with anaemia in a patient taking an NSAID or aspirin warranting iron studies.
Although many dispense events in pharmacies are routine, there are constant checks to ensure the appropriate use of medicines, he adds, including confirming previous doses of medicines and indications for use, as well as ensuring appropriate adherence to the regimen.
‘The availability of pathology results will provide another method of checking the appropriateness of a person’s medication regimen,’ Dr Tenni says. ‘As with the more routine checks undertaken, it may be more appropriate to seek pathology results when a new medication, new dose of medication or a symptom prompts investigation.’
References
- Therapeutic Goods Administration. Medicine shortage reports database. 2024. At: apps.tga.gov.au/Prod/msi/search?shortagetype=All
- Weekes L, Razman I. Prescription of compounded ophthalmic medications – a pharmacy perspective. Clin Exp Optom 2021;104(3):406–411.
- Therapeutic Goods Administration. Search warrant executed on South Yarra pharmacy. 2024. At: www.tga.gov.au/news/media-releases/search-warrant-executed-south-yarra-pharmacy.
- Therapeutic Goods Administration. Search warrant executed on Sydney residence in relation to substandard compounded semaglutide. 2024. At: www.tga.gov.au/news/media-releases/search-warrant-executed-sydney-residence-relation-substandard-compounded-semaglutide
- Worthington E. Melbourne compounding pharmacy raided as part of probe into alleged copycat Ozempic manufacturing. 2024. At: www.abc.net.au/news/2024-03-01/melbourne-compounding-pharmacy-raid-ozempic-weight-loss-drugs/103528992
- Worthington E, Robinson L, Wiggins N. ABC. Four Corners. The hunt for the Australian ‘coyboy’ pharmacist behind a replica Ozempic and Mounjaro scam. 2024. At: www.abc.net.au/news/2024-04-01/cowboy-pharmacist-behind-a-replica-ozempic-and-mounjaro-scam/103644794
- Worthington E. Patients report alarming side effects after injecting Ozempic and Mounjaro. 2024. At: www.abc.net.au/news/2024-05-14/replica-ozempic-and-mounjaro-side-effects/103654198
- Australian Government, Ministers, Department of Health and Aged Care. Protecting Australians from unsafe compounding of replica weight loss products. 2024. At: www.health.gov.au/ministers/the-hon-mark-butler-mp/media/protecting-australians-from-unsafe-compounding-of-replica-weight-loss-products?language=en
- Sansom LN, ed. Australian pharmaceutical formulary and handbook. 2024. At: https://apf.psa.org.au
- Border Watch. H Glover chemist and druggist. 1899. At: nla.gov.au/nla.news-article81713454



 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more




