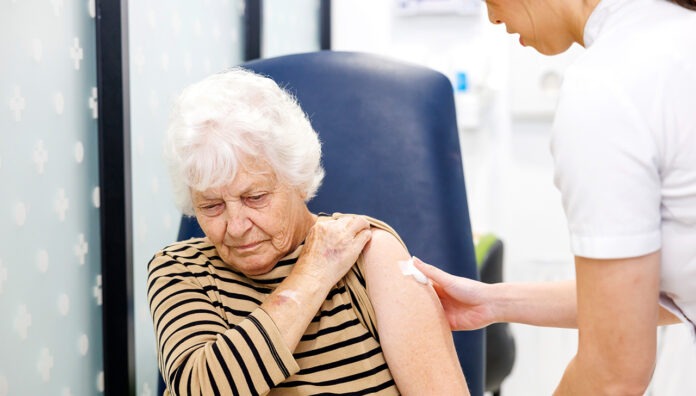
From guiding older patients on National Immunisation Program (NIP) stock to clarifying second-dose rules, here’s what pharmacists need to know about the 2025 influenza season.
1. Patients aged 65 years and older should wait for NIP stock to arrive
By this time of the year, most pharmacies will have ordered and received private stock of influenza vaccines. But for the 2025 season, deliveries of NIP are expected to commence around late March, following the confirmation of pre-allocated orders by pharmacies.
Older patients who present to the pharmacy requesting an influenza vaccine should be advised to wait until NIP stock arrives for optimum protection.
Patients who are 65 years and over should receive the NIP-funded Fluad Quad
0.50 mL vaccine or Fluzone High-Dose Quadrivalent, adjuvanted quadrivalent vaccines designed to boost the immune system’s response to the vaccine.
These vaccines help to generate a stronger and more sustained antibody response, providing better protection against influenza and its complications in this vulnerable age cohort – reducing hospitalisations and severe outcomes from influenza.
2. Patients (mostly) only need one dose of an influenza vaccine
If a patient received an influenza vaccine earlier on in the season and is concerned about waning immunity – one vaccine is still enough. Currently, there is insufficient evidence to justify routinely administering a second influenza vaccine dose within the same season.
Optimal protection from the influenza vaccine persists for around 3–4 months after vaccination. While the vaccine’s effectiveness begins to wane after this point, most patients should be sufficiently protected throughout the season.
However, there are some exceptions. Patients eligible for a second dose include:
- children aged 6 months to less than 9 years receiving their influenza vaccine for the first time
- people receiving an influenza vaccine for the first time following a haematopoietic stem cell transplant or solid organ transplant.
Patients travelling abroad may also benefit from an additional influenza vaccine dose within the same year, especially if visiting the Northern Hemisphere during its flu season (October to May).
If a patient recently had a 2024 formulation of influenza vaccine, for example in late 2024 or early 2025, they are also recommended to receive a 2025 formulation of influenza vaccine.
3. The best time to get vaccinated is before the flu season starts
The Australian influenza season typically runs from May to October, peaking in August.
And this season looks set to be a doozy. There have already been 31,289 confirmed cases of influenza reported this year, more than the number of cases reported in quarter one last year (30,497) – despite 2024 recording the highest number of influenza cases since surveillance began in 2001.
4. There is no combination vaccine available – yet
In mid 2024, Moderna reported positive results from the phase 3 clinical trial of mRNA-1083 – a combination influenza and COVID-19 vaccine.
According to Moderna, the combined vaccine was reported to generate a higher immune response against COVID-19 and three influenza strains when compared to co-administered vaccines.
Other pharmaceutical giants, including Pfizer and BioNTech are also undergoing late-stage trials for combined COVID-19 and flu vaccines.
Locally, Mater Researchers in Brisbane launched a trial of a non-mRNA combined COVID-19 and flu vaccine in October 2024. Developed by Novavax, the vaccine contains part of the coronavirus spike protein and aims to protect people who cannot have mRNA vaccines.
As we wait for these vaccines to hit the market, pharmacists should encourage co-administration of influenza and COVID-19 vaccines in at-risk groups, including:
- patients aged 75 years and over
- patients aged 65–74 years
- patients aged 18–64 with severe immunocompromise.
5. The conditions for the influenza vaccine are the same under the NIP
The NIP continues to provide free influenza vaccines for:
- children aged 6 months to less than 5 years
- patients aged 65 years and older
- Aboriginal and Torres Strait Islander people aged 6 months and older
- pregnant people
- people aged 6 months and older with certain medical conditions that increase their risk of severe influenza complications, as per the Australian Immunisation Handbook.
However, as per the ATAGI statement, there is an expansion of the NIP-funded influenza vaccine options for certain at-risk groups.
The cell-based influenza vaccine Flucelvax Quad is now funded under the NIP for patients aged 5-64 years at risk of influenza complications, including:
- Aboriginal and Torres Strait Islander people
- pregnant women
- people who have certain medical conditions.
For more information on the 2025 influenza vaccination season, don’t miss the PSA member briefing on the ATAGI 2025 seasonal influenza vaccination advice, this Wednesday 12 March from 7pm AEDT .



 This article was sponsored and developed in collaboration with PSA and Carers NSW[/caption]
However, pharmacists may perceive medication errors or non-adherence as a carer’s inability to fulfil this role,
This article was sponsored and developed in collaboration with PSA and Carers NSW[/caption]
However, pharmacists may perceive medication errors or non-adherence as a carer’s inability to fulfil this role,

 Now a PhD candidate, former Sudanese refugee and NSW Pharmacist of the Year
Now a PhD candidate, former Sudanese refugee and NSW Pharmacist of the Year  David North OAM
David North OAM NSW Early Career Pharmacist of the Year Lily Pham
NSW Early Career Pharmacist of the Year Lily Pham