One week into the rollout of the COVID-19 vaccine, Australian Pharmacist looks at the progress so far, and what is yet to come.
To date, 41,907 doses of the Pfizer vaccine have been administered under Phase 1a of the national rollout strategy.
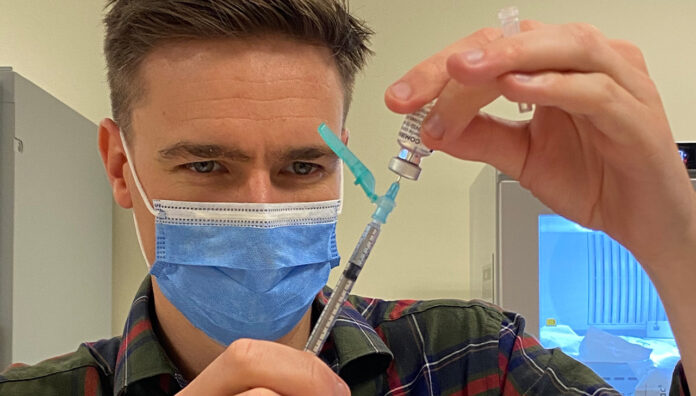
Pharmacists across the country are playing a vital role in the effort, including Steven Walker, Lead Pharmacist Workforce and Development at Alfred Health in Melbourne, who has been busy helping with the administration of the COVID-19 vaccine to hospital staff.
‘Our motto in our department is that we’re happy to do our part by rolling up our sleeves to receive the vaccine,’ he told Australian Pharmacist
‘I’ve had my first dose, and it’s really a privilege to be a part of the first group to receive the vaccine.’

To prepare for delivery, the Alfred team went through a rigorous training program which focused on aseptic technique and running through simulations.
‘We ran simulations of every step of the process and advised pharmacists how to perfect their technique,’ Mr Walker said.
‘We figured out what the ingredients and excipients of the Pfizer vaccine are and simulated the receipt of the vials, putting them into the freezer, exchanging from the freezer to the fridge, and went through the motions of actually preparing the vaccine.’
To prevent waste, the Alfred pharmacists are using appropriate aseptic technique and following the procedures they created.
‘It helped to provide each other advice in the pre-training stage about the appropriate processes for drawing out the required dose,’ Mr Walker said.
‘Having the right equipment is also important, along with ensuring you’re utilising consumables, syringes and needles effectively to get the most efficient preparation of the vaccine.’
Factoring in the timings to maintain cold chain and effectively transfer the vaccine to the refrigerator is also an instrumental part of the process.
‘We will also typically determine the number of doses we need to put into the refrigerator at a reasonable timeframe prior to our clinic sessions,’ Mr Walker added.
Some members of the Alfred team are now preparing to help administer the vaccine to quarantine hotel workers.
‘Most of our pharmacists who will be involved in the vaccination of these frontline workers were deployed to the hotels on 2 March,’ Mr Walker said.
The Alfred pharmacists stationed in hotel quarantine will be involved in preparing the vaccine for nurses and other trained immunisers to deliver, but once the vaccination effort ramps up the team is fully prepared for delivery.
‘We put our pharmacists through the PSA immunisation training course to become approved pharmacy immunisers so the ability to deliver the vaccine is built into their capacity,’ Mr Walker said.
What pharmacists need to know
On 1 March, 300,000 doses of the Oxford-AstraZeneca vaccine arrived in Australia. The doses are currently being batch-tested by the Therapeutic Goods Administration (TGA), with 200,000 set to be released to the states for rollout from Monday (8 March).
The arrival of the AstraZeneca vaccine has more than doubled the number of COVID-19 vaccine doses in Australia, Federal Minister for Health and Aged Care Greg Hunt announced in a statement last week.
‘We will now be able to scale up the vaccination rollout to our priority groups, including our most vulnerable Australians and to our frontline border and health workers,’ he said.
By late March, the CSL Australian-produced AstraZeneca vaccines should be ready, with 2 million expected by month’s end.
Community pharmacists who submitted an expression of interest to deliver the vaccine should complete the COVID-19 vaccination training program.
To assess if presenting patients belong to a priority group, pharmacists should use the Healthdirect eligibility checker or contact the National COVID-19 Helpline, where call handlers will be trained to answer questions about eligibility.
To confirm if patients have contraindications, pharmacists should check the product information for both the Pfizer and AstraZeneca vaccines on the TGA website, and follow usual clinical protocols, along with checking patient information on My Health Record (MHR) and the Australian Immunisation Register (AIR).
Pharmacists will also be able to check if the COVID-19 vaccine has already been administered to a patient through their MHR and AIR. Once a patient has received the vaccine, it should be recorded in their AIR within 24 hours.
While the COVID-19 vaccine is free for all Australian citizens, permanent residents, and most visa holders, patients not eligible for Medicare should be advised to visit a Commonwealth-funded GP Respiratory or territory vaccination clinic.
Pharmacies that provide vaccinations to non-eligible patients will not receive funding for the delivery of these services.
Promoting vaccine services
There are several parameters around promoting the availability of the vaccine that pharmacists must follow. The TGA has advised that health professionals must not use self-developed advertising or risk breaching prohibitions for promoting prescription medicines.
Pharmacists are able to use government-produced materials across all communication channels including websites, social media, and flyers and posters to inform customers that they will be delivering the COVID-19 vaccine in their practice.
They are able to add factual information, including:
- the location of the COVID-19 vaccination service
- opening hours for the vaccine clinic
- whether an appointment is necessary and how to make one.
If using government material, pharmacists must ensure that they:
- are genuine and unaltered, aside from the addition of factual details
- do not promote a take-home message through their proximity to other promotional materials
- do not include the trade name or active ingredient of the vaccine, or make any comparisons between the different vaccines
- do not imply that patients risk harm if they do not receive the vaccine or incentivise to patients to obtain it.
However, providing factual and balanced information, such as a view of vaccination in general, technical details about the development of vaccines and sharing scientific reports, likely won’t be considered as unlawful advertising.
For more information on COVID-19, see PSA’s microsite.


 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more