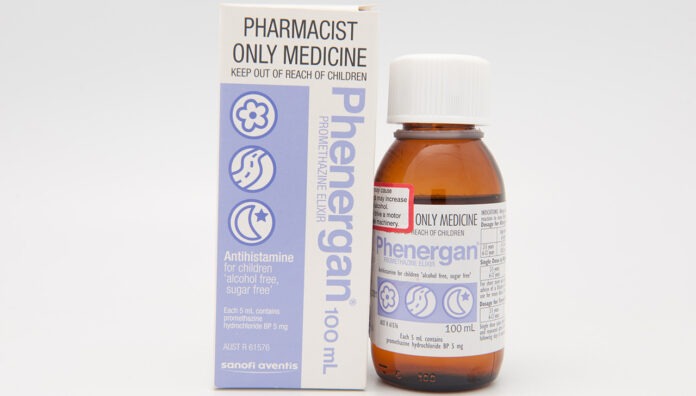
The Therapeutic Goods Administration (TGA) has worked with Phenergan manufacturer Sanofi to develop a new contraindication for the medicine in children under 6.
The decision to restrict use of the medicine in this age cohort – commonly used to treat allergies, travel sickness and nausea – is based on a thorough evaluation of new scientific evidence that the risks outweigh the benefits, a spokesperson for Sanofi told Australian Pharmacist.
‘Following recent evaluations made by Sanofi Consumer Healthcare Pty Ltd, including a benefit-risk assessment, the research has shown a causal association between promethazine and safety concerns,’ said this spokesperson.
The sponsor-initiated update was prompted by advice from the TGA Advisory Committee on Medicines (ACM) – first-generation oral sedating antihistamines – do not use in children, said a spokesperson from the TGA.
‘Further review by the sponsor of cumulative safety data in children 2 to 5 years of age demonstrated sufficient evidence of a causal association between promethazine and psychiatric and central nervous system adverse events in this age group,’ the TGA spokesperson told AP.
The contraindication comes after Medsafe, the New Zealand Medicines and Medical Devices Safety Authority, moved to contraindicate the use of the medicine in children under 6 due to the potential for fatal respiratory depression, psychiatric and central nervous system (CNS) events.
AP understands that the contraindication will affect other products. A spokesperson for AFT said a variation has been submitted to update the product information and label on Allersoothe Elixir with a change to the age indication from children 2 years and older to children 6 years and older. This submission is pending approval.
Will the packaging change?
Not immediately.
New labels for promethazine-containing products will be rolled out in 2025 due to production and logistics lead times, said the Sanofi spokesperson.
‘No immediate action is required regarding current stock. There is no need to set aside existing medications, as this communication does not constitute a recall,’ said the spokesperson. ‘Similarly, the revised labels do not create any changes in the ordering process.
The product information for Phenergan has been updated and is available here.
What should pharmacists do if they get a Phenergan script for an 18 month old infant?
The use of promethazine in children under 6 years of age is now both contraindicated and off label, said Peter Guthrey MPS, PSA Senior Pharmacist – strategic policy.
‘This means that if you choose to dispense, both the prescriber and dispenser carry the full indemnity risk, and you need to get the patient to provide informed consent,’ he said.
Should a parent present with a script for promethazine for use in a child under 6 years of age, pharmacists should contact the prescriber to discuss the risks and benefits, noting that it’s contraindicated.
Is there any situation where it would be appropriate to dispense Phenergan to a young child?
It would need to be a pretty compelling case, where the benefit to a child outweighed the risk of respiratory depression, psychiatric or CNS events, said Mr Guthrey.
‘Given it’s a documented contraindication, it’d be pretty hard to demonstrate the medicine is ‘‘safe and therapeutically appropriate’’,’ he said.
‘It is going to be difficult to find a situation where supply is clinically justified and pharmacists have met their obligations to patient safety.’
What about prescribing Phenergan as a Schedule 3 medicine?
If a parent requests promethazine as a Schedule 3 medicine for someone under the age of 6 years, generally alternate products or advice for treatment should be provided following a discussion.
‘While a pharmacist prescribing promethazine as a Pharmacist Only Medicine is lawful for children aged 2-5 years, it is still contraindicated,’ said Mr Guthrey. ‘There would be very few, if any, situations where prescribing the medicine where contraindicated would be warranted.’
What about phenylephrine?
There are some combination phenylephrine products indicated for children under 6 years of age for nasal congestion. However, there is plenty of evidence showing that oral phenylephrine products are ineffective as a nasal decongestant.
In fact, the United States Food and Drug Administration (FDA) recently announced it will move to ban oral phenylephrine products because they simply don’t work.
‘Based on our review of available data, and consistent with the advice of the advisory committee, we are taking this next step in the process to propose removing oral phenylephrine because it is not effective as a nasal decongestant,’ said Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research.
While a spokesperson for the TGA told Australian Pharmacist back in July that there are ‘no plans’ to review the effectiveness of oral phenylephrine, it will continue to monitor the outcomes of the FDA review.
What advice should pharmacists provide?
For children with allergies, non-sedating antihistamines are first line.
The best thing parents can do when a child under 6 years of age has cough or cold symptoms is to wait it out, with a review by a medical practitioner when appropriate.
‘It’s about reassurance to parents that the cough in itself isn’t harmful, and that kids don’t always need medicines for a cold – they need time and supportive therapy,’ said Mr Guthrey.
It’s important that pharmacists give parents confidence that they’re doing the right thing by not treating the cough, and providing symptomatic relief for fever and chills – ensuring they are appropriately counselled on when review by a medical practitioner may be appropriate.
For more information, refer to the Australian Pharmaceutical Formulary and Handbook treatment guidelines on:


 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more