A megatrend has begun in medicines. The increasingly large and full fridges found in pharmacies stand as a testament to the growing prevalence of injectable medicines – and are a sign of things to come.
In the near future, many daily tablets may be replaced with periodic appointments to have a medicine administered by injection.
The injectables are coming
Options for injectables and other non-oral dose forms such as patches, gels and sprays are rapidly growing, with these increasingly replacing medicinal therapies delivered through swallowing tablets, capsules or liquids. Pharmacists will recognise these examples.
Prolia (denosumab), which has been registered for the treatment of osteoporosis in Australia since 2010, offers a glimpse of the future, replacing daily or weekly tablets with biannual injections.1 Similarly, the relatively new cholesterol-lowering drug Leqvio (inclisiran) is administered every 6 months as a maintenance dose, after the initial induction doses, offering a new route for lipid modifying therapy.2
And Eli Lilly has a new US Food and Drug Administration-approved injectable, Kisunla (donanemab-azbt), coming out for the treatment of Alzheimer’s disease (AD).3 Delivered by infusion every 4 weeks, the drug works by targeting deposits of amyloid plaque in the brain, which is a key indicator of the presence of the debilitating, ultimately fatal brain disease.
Last month, one of the world leaders in AD research, Professor Colin Masters of The Florey Institute of Neuroscience and Mental Health in Melbourne, told neurologists and healthcare workers who treat patients with progressive neurodegenerative diseases of promising results from the Roche Dominant Inherited Alzheimer’s Network (DIAN) trial of the monoclonal antibody gantenerumab. Roche’s “Brain Shuttle” technology is now being used to cross the blood-brain barrier and then clear amyloid plaque aggregation with a newer generation, bio-specific, monoclonal antibody called trontinemab.
Prof Masters told a Creutzfeldt-Jakob Disease Support Group Network (CJDSGN)workshop on CJD and other prion diseases that early results from a trial of trontinemab are currently in pre-press in The Lancet.⁴
It’s also hoped, he says, that current intravenous AD drug models may evolve to be delivered subcutaneously via an autoinjector by patients at home.
Perhaps most revolutionary, however, are the advancements in HIV prevention. Recently, research into Gilead’s long-acting injection, lenacapavir (an agent for treatment of HIV), has shown promising results in pre-exposure prophylaxis (PrEP), and has been described as ‘the closest we have ever been to an HIV vaccine’ .⁵ At the AIDS 2024 conference in Germany in July, researchers presented their findings that twice-yearly lenacapavir demonstrated 100% efficacy for HIV prevention in cisgender women.⁶
‘The pace at which innovative, new medicines are entering the market is unprecedented,’ says Medicines Australia CEO Liz de Somer. ‘Advances in research, technology and innovation mean we can expect to see new forms of treatments – which include innovative new medicines and improved forms of existing medicines – becoming available.’
These are huge leaps forward in how health is treated, and pharmacists will need to adapt, says PSA National Board member Dr Shane Jackson FPS, community and credentialed pharmacist and lecturer in pharmacy at the University of Tasmania.
‘Medicines have always been an evolving space. Research on mRNA-delivered therapeutics has actually been around for about 20 years, and the pipeline of mRNA therapeutics is and will be a gamechanger in terms of treatment and prevention of disease in the future,’ he says.
‘What this means is that pharmacists will be needed more than ever because of their deep expertise in the use of medicines, and the term “medicines experts” will most likely evolve to “therapeutics experts”, noting the change from “medicine” to the complete management and prevention of disease.’
 New formats needed
New formats needed
One reason alternative delivery systems like injections and patches are becoming more prevalent is because they are critical for delivering new, biologically complex drugs.
As scientists have developed new treatments, they have created molecules that are too large or sensitive to be absorbed effectively through the digestive system. For example, Humira (adalimumab), the monoclonal antibody used to treat autoimmune conditions including juvenile arthritis and Crohn’s disease, is administered through subcutaneous injection because its large molecular structure would be ineffective if taken orally.⁷
Or take the testosterone transdermal delivery patch Androderm.8 Delivering testosterone directly through the skin provides a steady hormone level that can’t be achieved with oral testosterone.⁹
Technological advancements in mRNA technology, showcased in the production of COVID-19 vaccines, are also driving treatments and other preventive measures for different conditions.
‘Prior to the pandemic, mRNA-based drug products were focused primarily on treating oncology indications as opposed to infectious diseases, such as COVID-19,’ says Martin Gonzalez, Pfizer’s senior manager of product development.10
‘However, technical and scientific advancements have allowed researchers and drug developers to expand the use of mRNA to new therapeutic areas.’
For patients who inject themselves daily, there is also a growing preference for better ways to self-administer medicines. This focus on the patient has led to advances in medical devices in recent decades, introducing prefilled syringes, pens and automated electronic and infusion devices.

‘Much of the emphasis in contemporary drug design has shifted from just preserving basic quality attributes, such as safety, efficacy and potency, in a simple container,’ Mr Gonzalez says.10
‘Today’s [sterile injectable] drugs carry a more complex profile and incorporate new thinking about ways of preserving the value of the drug while also providing additional benefits, including precise, easy-to-administer delivery systems for better dose compliance.’
Improving medicine adherence
According to the World Health Organization, around 50% of patients with chronic illnesses in developed countries don’t take their medicines as prescribed.11
The rates are even lower in developing nations. There are myriad reasons for poor medicine adherence, including low health literacy, the prescription of complex drug regimens and limited access to care.12
Long-acting therapies may also be beneficial for hard-to-reach populations, such as those experiencing homelessness or substance use problems. And in the case of those living with HIV, long-acting drugs might also be useful for children, says Dr Charles Flexner, HIV expert at Johns Hopkins University. Globally, just 50% of children diagnosed with HIV are receiving treatment, in part due to a lack of drug versions made for children.13
‘With long-acting formulations, that will no longer be the case,’ Dr Flexner says.13 ‘Children will be able to use the same formulation as adults, just at a different dose.’
Breakthrough in injectable PrEPLong-acting injectables for HIV treatment and prevention are providing convenience, promoting better adherence, and helping to reduce the stigma and discrimination that might come with taking a daily pill.14 In Australia, there is currently one injectable HIV medicine listed on the Pharmaceutical Benefits Scheme (PBS) – Cabenuva (cabotegravir/rilpivirine), which requires injections every 2 months.15 Cabotegravir-LA for pre-exposure prophylaxis (PrEP) has been approved by the Therapeutic Goods Administration (TGA) but is not yet listed on the PBS,16 and lenacapavir is also TGA-approved but not PBS listed for treating multidrug-resistant HIV in combination with other antiretroviral therapy. Globally, 6-monthly injections of lenacapavir have also been trialled for PrEP, with extraordinary results.
Trials have also shown an efficacy rate of 100% among cisgender women in South Africa and Uganda. Professor Sharon Lewin, Director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne, and immediate past president of the International AIDS Society, described the results as ‘a breakthrough advance with huge public health potential’. ‘If approved and delivered – rapidly, affordably and equitably – to those who need or want it, this long-acting tool could help accelerate global progress in HIV prevention,’ Professor Lewin said.⁶ When it comes to long-acting injectables for HIV treatment and prevention: watch this space. |
Pharmacist focus on patient care
As more injectable medicines come onto the market, the pharmacy of the near future will need more consultation rooms and more dispensary refrigerator space.
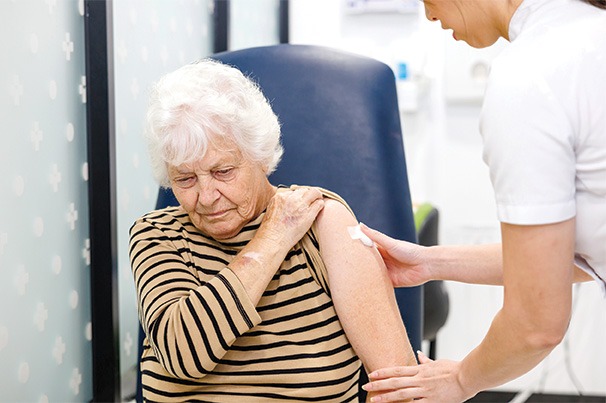
Pharmacy practice will likely involve more direct patient care, including administering injections and managing complex treatment regimens. Those who fail to adapt, for example by not learning to inject safely, risk becoming obsolete, as the profession increasingly shifts to a more hands-on role.
‘In terms of medicines administration [by injection], this is a newish activity that pharmacists are undertaking,’ Dr Jackson points out. ‘It is becoming increasingly relevant because of a lack of access to general practice care and the acknowledgement that this activity is and has always been within the scope of practice of a pharmacist in terms of education and training.
‘Pharmacists need to ensure they are competent in terms of their training (vaccination certified, medicines administration training has been completed) and that they inform themselves of the relevant information related to each drug being administered.
 ‘In my view, it’s analogous to dispensing a new drug that you haven’t come across before. You understand the relevant information related to the drug from a safety and effectiveness point of view, you communicate with the patient regarding relevant counselling points, and you document relevant information.’
‘In my view, it’s analogous to dispensing a new drug that you haven’t come across before. You understand the relevant information related to the drug from a safety and effectiveness point of view, you communicate with the patient regarding relevant counselling points, and you document relevant information.’
But the future isn’t just about the administration of a medicine. Rather, Dr Jackson says, this is just the start of pharmacists ‘being more involved and taking more responsibility and accountability for patient care’.
‘Regulatory misunderstanding and regulatory impediments have for a long time prevented pharmacists from doing more in the prescribing, administration and review of medicines.
‘We are seeing these barriers being reduced, albeit slowly in some jurisdictions, to allow pharmacists to do more, to practise to their full scope in terms of medicines management.
‘Ideally, pharmacists should be empowering patients to better take control of their own health care. For most of the medicines we see currently, patients with appropriate support can self-administer,’ says Dr Jackson.
‘In some cases, there may be barriers in place and pharmacists may deliver regular injections for these patients. It’s a case-by-case basis with the patient at the centre of care.’
References
- Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in postmenopausal women and men over 50 years of age. 2024. At: www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/pharmacologic-approaches-to-prevention/denosumab
- NPS Medicinewise. Leqvio. 2023. www.nps.org.au/medicine-finder/leqvio
- US Food and Drug Administration. FDA approves treatment for adults with Alzheimer’s disease. 2024. At: www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-adults-alzheimers-disease
- Bateman RJ, Li Y, McDade E, et al. Amyloid reduction and dementia progression in dominantly inherited Alzheimer’s disease after long-term gantenerumab treatment: results from the Dian-Tu trial. Lancet 2024. Epub 2024 Jul 26.
- Lay, K. HIV drug could be made for just $40 a year for every patient. The Guardian. 2024. At: www.theguardian.com/global-development/article/2024/jul/23/hiv-aids-prevention-vaccine-lenacapavir-sunlenca-pharmaceuticals-gilead-generic-licensing
- International AIDS Society. Statement from IAS President Sharon Lewin on full results from PURPOSE 1 trial of twice-yearly injectable lenacapavir for HIV prevention. 2024. At: www.iasociety.org/news-release/statement-ias-president-sharon-lewin-full-results-purpose-1-trial-twice-yearly
- Ellis CR, Azmat CE. StatPearls. Adalimumab. 2023. At: www.ncbi.nlm.nih.gov/books/NBK557889/
- NPS Medicinewise. Androderm. 2020. www.nps.org.au/medicine-finder/androderm-transdermal-patch
- Shoskes JJ, Wilson MK, Spinner ML. Pharmacology of testosterone replacement therapy preparations. Trans Androl Urol 2016;5(6):834–43.
- Gonzalez M. Recent trends and developments in injectable drug formulation and delivery. ONdrugDelivery. 2022;133:16–18. At: www.ondrugdelivery.com/recent-trends-and-developments-in-injectable-drug-formulation-delivery/
- World Health Organization. Adherence to long-term therapies: evidence for action. 2003. At: iris.who.int/handle/10665/42682
- Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4): 304–314. At: www.ncbi.nlm.nih.gov/pmc/articles/PMC3068890/
- Mandavilli A. Long-acting drugs may revolutionize HIV prevention and treatment. The New York Times. 2024. At: www.nytimes.com/2024/04/17/health/hiv-long-acting-shots-pills.html
- ACON. Long-acting injectables for HIV treatment and prevention. 2021. At: https://www.acon.org.au/wp-content/uploads/2021/08/2021-08-20-ACON-Long-acting-injectables-for-HIV-treatment-and-prevention-.pdf
- ASHM. Long-acting injectable HIV treatment tool. ashm.org.au/wp-content/uploads/2024/08/ASHM-Long-Acting-Injectable-HIV-Treatment-Tool-Aug24.pdf
- ASHM. Implementing long-acting injectable HIV pre-exposure prophylaxis using Cabotegravir-LA: recommendations to support the Australian health sector. 2024. At: ashm.org.au/wp-content/uploads/2024/09/Implementing-long-acting-injectable-HIV-pre-exposure-prophylaxis-using-Cabotegravir-LA_2024_Summary-Report.Final_.pdf
- AVAC. Second pivotal trial of twice-yearly HIV prevention injection safe and highly effective: PURPOSE 2 trial among gay men, trans and nonbinary people. 2024. At: avac.org/press-release/purpose-2/
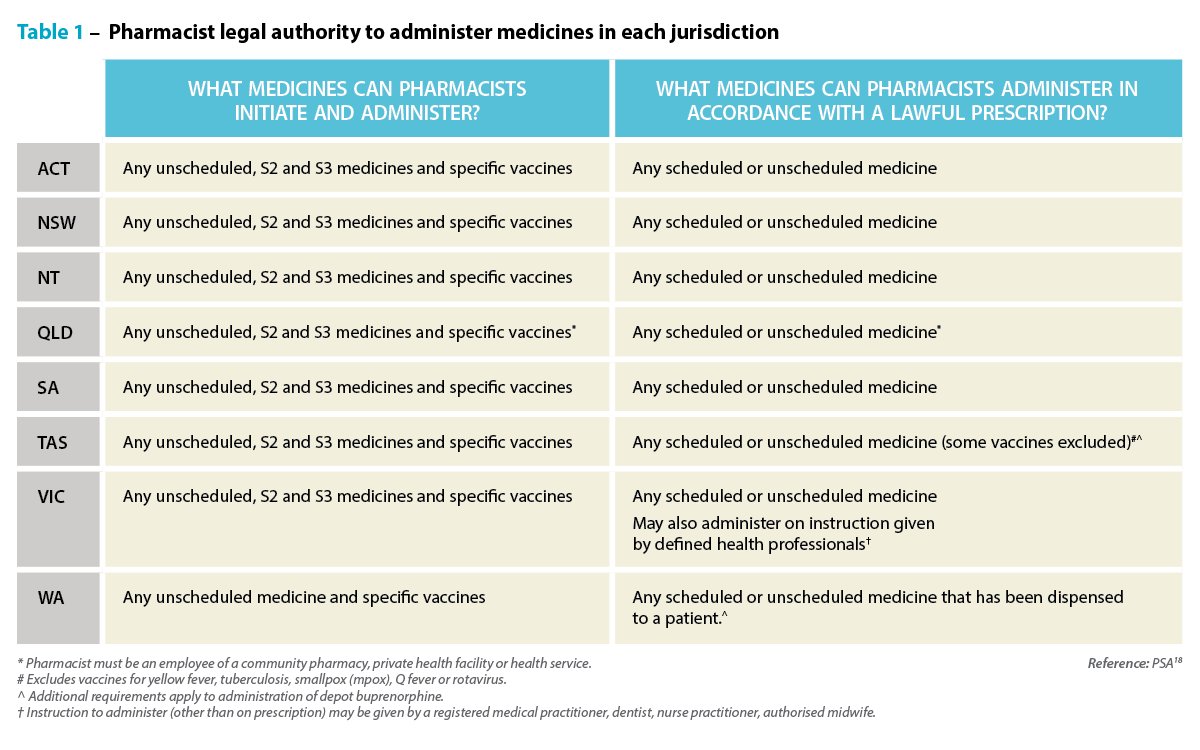
- Pharmaceutical Society of Australia. Administering medicines by injection. At: www.psa.org.au/programs/administering-medicines-by-injection/



 Professor Stephen Nicholls[/caption]
Professor Stephen Nicholls[/caption]




 From centre: PSA SA/NT President Manya Angley FPS, Matthew Gillespie and Minister for Health and Wellbeing Chris Picton MP[/caption]
From centre: PSA SA/NT President Manya Angley FPS, Matthew Gillespie and Minister for Health and Wellbeing Chris Picton MP[/caption]

 Pooja Jadeja MPS[/caption]
Pooja Jadeja MPS[/caption]

 Clinical trial data released this year in a study of cisgender men, transgender men, transgender women, and nonbinary individuals who have sex with partners assigned male at birth showed that using lenacapavir results in a 96%
Clinical trial data released this year in a study of cisgender men, transgender men, transgender women, and nonbinary individuals who have sex with partners assigned male at birth showed that using lenacapavir results in a 96% 



