In introducing the new medicinal cannabis regulatory framework last year, the Government’s intent was to provide patient access to Australian-grown and manufactured medicinal cannabis products, initially outside the standard registered medicines route.
Australia differs from countries such as the US and Canada in that the scheme is centred on providing a product made to medicines quality standard, through a doctor’s prescription. The inclusion of a quality standard recognises that ‘home-made’ cannabis extracts are from uncontrolled sources and are often of varying strengths and may even lack any active ingredient. Cannabis products obtained through poorly regulated sources may contain a range of potentially serious microbial and fungal contaminants.1 Fungally contaminated medicinal cannabis is thought to have caused the death of one and possibly two Californians earlier this year.2,3
The Government’s second decision was that medical practitioners and pharmacists must have a central role in the prescription and dispensing of medicinal cannabis – in this sense cannabis should be treated like other medicines, including unapproved medicines. At the same time, the new regulatory framework encourages pathways to clinical trialling of products and if the trials are successful, for future TGA registration of suitable products.
Who are the regulators?
The Commonwealth Department of Health includes the two Commonwealth ‘arms’ of the medicinal cannabis scheme. Cultivation and manufacture of medicinal cannabis products is regulated by the Office of Drug Control, which also manages import and export of a range of drugs products through administration of relevant Customs regulation. Standards for product quality, pharmaceutical product manufacture (GMP requirements), medicinal cannabis and cannabinoid scheduling and patient access schemes for cannabis products (with the exception of nabiximols, or Sativex, which is on the ARTG but not marketed in Australia, they are unapproved medicines) is through the Therapeutic Goods Administration (TGA).
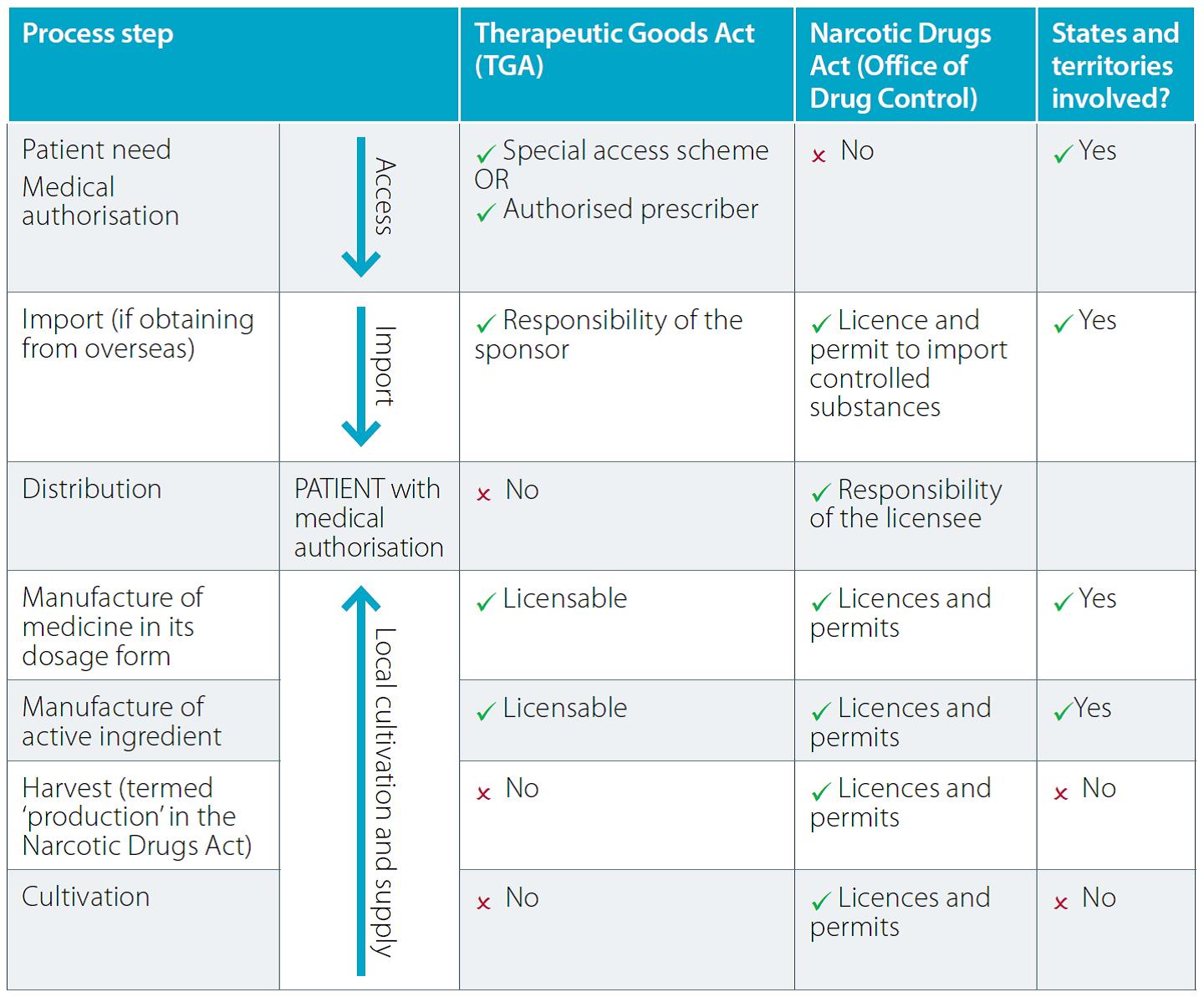
States and territories also have a significant role in regulating medicinal cannabis products (Table 1).
This derives from their role as the regulators of pharmacies and medicines handling and distribution within their jurisdictions. The distribution of medicinal cannabis is also regulated by the states and territories. Schedule 8 medicines (which include cannabis and many cannabis-based products) are thus also subject to state and territory regulation, while some states such as Queensland and Victoria have enacted specific legislation around the prescribing and dispensing of medicinal cannabis products. These can extend to cannabidiol products, which if sufficiently free from other cannabinoids are in Schedule 4 rather than 8.
The implementation of medicinal cannabis legislation does not involve the legalisation of recreational cannabis. Australia is a signatory to the Single Convention on Narcotic Drugs 1961 which aims to combat drug abuse through coordinated international action. The Narcotic Drugs Act 1967 provides the Commonwealth with powers to meet Australia’s obligations under the Single Convention. This includes regulation of narcotic drug manufacture and cannabis cultivation for medicinal and related scientific purposes.
Cultivating medicinal cannabis
A licence is required to cultivate cannabis for medicinal purposes, and with the risk of illegal diversion of cannabis both the Narcotic Drugs Act and Regulations impose strict requirements, including:
- Persons and entities involved in medicinal cannabis cultivation or manufacture (must meet a ‘Fit and Proper Persons Test’, employee suitability requirements and financial viability requirements)
- Location and security of medicinal cannabis cultivation or manufacturing sites (cultivation does not have be in an indoor or glasshouse system, but certain security principles must be met in all cases)
- Storage, handling, transport and destruction of medicinal cannabis
- Record keeping and auditing of medicinal cannabis distribution activity.
As of 6 July 2017, 13 medicinal cannabis licences had been issued, including 8 commercial and 5 research licences.
Permits are issued in relation to a licence. These set out details such as amounts and strains of cannabis needed before cultivation of particular crops can begin, and three permits had been issued as of 6 July 2017. Demand and supply are linked in the regulatory process. For permits to be issued to Australian manufacturers, there needs to be communication between health professionals seeking to obtain access for patients and medicinal cannabis manufacturers, with some guarantee that production will not be excessive.
To safeguard against the presence of contaminants, and to enable confidence in the information on the product labels on concentrations of active ingredients, products must conform with Therapeutic Goods Order 93 (Standard for Medicinal Cannabis). The Order applies to both imported and Australian manufactured products.
Export of medicinal cannabis products is not currently permitted. However, in June 2017, the Minister for Health asked the Office of Drug Control to explore options to allow the export of medicinal cannabis products while simultaneously enabling sufficient product to be made available for domestic use. The Government is committed to ensuring the long-term availability of domestically grown medicinal cannabis products to Australian patients. At the same time, it is important not to constrain the growth of an emerging domestic industry through restricting market opportunities.
Import of ‘bulk sponsored’ supplies
In early 2017, processes were put in place to reduce the time it takes to physically get medicinal cannabis to patients, following approvals of prescriptions under the SAS B and Authorised Prescriber pathways. Instead of imports being required on a patient-by- patient basis, companies can import a range of products that are anticipated to be required for larger numbers of patients.
The material is kept under storage condition used for bulk Schedule 8 medicines and is imported through a licence issued under the Customs (Prohibited Imports) Regulations and subject to strict conditions. The approach is allowed because there is a check on the use of these products by TGA prior to prescribing. State or Territory wholesaling and distribution licences are also required for these products. A list of some of the importers and products is provided at: www.odc.gov.au/importers-andmanufacturers- medicinal-cannabis-products. Products available in Australia include THC and CBD oils, oromucosal solutions, CBD capsules and raw cannabis for vaporisation.
Scheduling
Scheduling of medicines and chemicals is a process of determining the levels of control on access that can be applied to a substance, based on risk. Each schedule has a different level of control about who can dispense them, how prescriptions must be made, labelling requirements etc.
Prior to 1 Nov 2016, most forms of medicinal cannabis were Schedule 9 prohibited substances. This made prescribing and patient access very difficult (and in some states impossible). Over the past year, there have been a number of changes to scheduling of medicinal cannabis (see www.tga.gov.au/schedulingdelegates- final-decisions). As of July 2017, cannabis containing tetrahydrocannabinols e.g. THC (extracts, or derivatives of extracts, of cannabis with a known psychoactive effect) for human therapeutic use are a Schedule 8 – Controlled drug, available only by prescription from a medical practitioner authorised by states and territories. The authorisation of the practitioner may require the ‘standard’ state Schedule 8 approvals, but in a number of jurisdictions there are additional medicinal cannabis approvals required for prescribing and dispensing.
Cannabis and its extracts or derivatives remain in Schedule 9 when not for human therapeutic use. The Schedule 8 entry for tetrahydrocannabinols allows for human therapeutic use under certain circumstances that are tightly defined and controlled. This includes that it must be extracted from cannabis. Nabilone, dronabinol (synthetic delta-9-THC) and nabiximols are also listed in Schedule 8.
Cannabidiol (CBD) in preparations for therapeutic use containing 2% or less of other cannabinoids found in cannabis are included in Schedule 4 (Prescription medicine). However, in some states (Queensland and Tasmania, and Victoria in certain circumstances), additional state approvals are required. As it is scheduled as a prescription medicine, CBD is not legal in cosmetic products, even if they do not make overt therapeutic claims. Nor are THC or CBD legal in food products.
In next month’s edition I give an update on the progress of reviewing the clinical efficacy of medicinal cannabis, and the development of clinical guidance documents for the potential use of medicinal cannabis in several major conditions.
Table 1. How the legislative requirements work together
References available upon request
DR JOHN SKERRITT is Deputy Secretary for Health Products Regulation, Commonwealth Department of Health, Canberra.



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more



 Sponsorship information
Sponsorship information


 Talking to patients who have questions
Talking to patients who have questions





