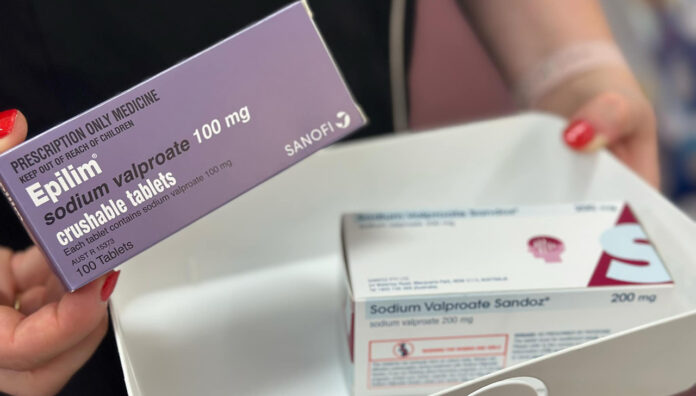
The Therapeutic Goods Administration (TGA) issued a caution over the use of sodium valproate in men who are planning a family.
Up to 1 in 10 babies (10%) exposed to sodium valproate during pregnancy may be born with birth defects.
One mother in the United Kingdom had two children born with fetal valproate spectrum disorder (FVSD) after using sodium valproate since the age of 16 following an epilepsy diagnosis.
Now, the anticonvulsant—used to manage seizures, mood disorders in bipolar patients, and as migraine prophylaxis—has been linked to an increased risk of neurodevelopmental disorders in children whose fathers used the medicine three months before conception.
These findings are based on a retrospective European observational study comparing the effects of the use of sodium valproate with those of lamotrigine or levetiracetam.
The neurodevelopmental disorders identified include autism spectrum disorder, intellectual disability, communication disorders and ADHD and movement disorders.
However, as of yet there have been no adverse events in relation to paternal exposure reported to the TGA.
What’s changing?
Women are already advised against using sodium valproate in pregnancy, which can cause FVSD. Now men are urged to consider alternative treatment options if planning to father a child or before discontinuing contraception.
New boxed warnings will also be introduced for Epilim. The Product Information (PI) and Consumer Medicine Information (CMI) will feature added warnings about paternal exposure.
Sponsors of generic sodium valproate products must update their safety information to match that of Epilim.
What should pharmacists do?
When dispensing sodium valproate to men, pharmacists should inform them about the risks of using this medicine if planning a family. The use of contraception should be encouraged in both the male patient and their female partner.
Pharmacists should also advise men to contact their GP if their partner is pregnant and they have taken sodium valproate within the 3 months before conception.
Male patients who are taking sodium valproate should not donate sperm for more than 3 months after ceasing treatment.
Pharmacists should recommend that men who have been taking sodium valproate long term have an annual review with the specialist to assess whether it remains the most appropriate treatment – particularly if they are planning on parenthood.



 This CPD activity is supported by an unrestricted education grant by Reckitt.[/caption]
This CPD activity is supported by an unrestricted education grant by Reckitt.[/caption]



 Jess Hadley, community pharmacist and Professional Officer at PDL[/caption]
Jess Hadley, community pharmacist and Professional Officer at PDL[/caption]
 Peter Guthrey, Senior Pharmacist – Strategic Policy at PSA[/caption]
Peter Guthrey, Senior Pharmacist – Strategic Policy at PSA[/caption]


 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]