
Two east coast states have made recent vaccine legislation updates, expanding community access through barrier removal.
From today (30 October 2023), a new Extended Practice Authority (EPA) announced by Queensland Health Minister the Hon. Shannon Fentiman MP will allow pharmacists to administer more vaccines to more patients across different healthcare settings.
What’s changed in Queensland?
The sweeping changes for Queensland means that pharmacists can now prescribe and administer:
- a wider range of vaccines
- vaccines in more settings, including general practice, residential aged care facilities and Aboriginal Community Controlled Health Organisations
- to patients of younger age, with a reduction to the minimum age for pharmacist administration dropped from 5 years to 2 years of age for most allowable vaccines. There is now no specific age restriction on pharmacist administration of Influenza and COVID-19 vaccines (appropriate guidelines should be followed).
What new vaccines can Queensland pharmacists prescribe and administer?
The following vaccines can now be administered by pharmacist immunisers in the approved settings or in outreach clinics under the EPA:
- Hepatitis B
- Varicella (chickenpox)
- Meningococcal B
- Human papillomavirus (HPV)
- Typhoid
- Zoster (herpes zoster)
- Japanese encephalitis.
Welcoming the changes, the PSA Queensland President Shane MacDonald MPS said the EPA demonstrated the government’s commitment to improving community access to healthcare services.
‘Allowing more people to be vaccinated by their local pharmacist is a vital step in not only protecting individual Queenslanders but the entire community,’ he said.
How will it impact patients?
Following a Vaccination Summit in Queensland earlier this month (20 October), the EPA changes were designed to remove legislative barriers to vaccine access.
Only around 30% of Queenslanders get vaccinated against influenza each year, with most 2023 diagnosed influenza cases (93%) occurring in unvaccinated patients. Queensland’s childhood vaccination rates have also dropped this year in comparison to the national average, including among Aboriginal and Torres Strait Islander children.
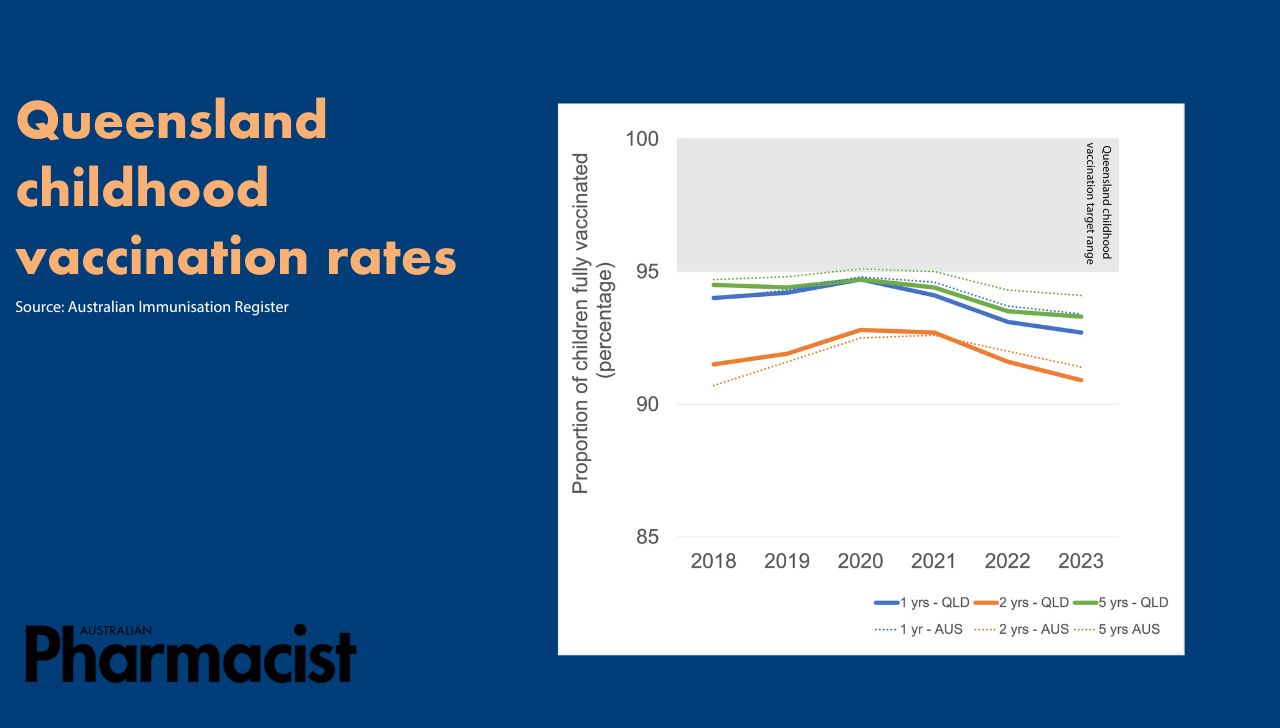 By ‘focusing on bringing the vaccine to the person rather than the person to the vaccine’, will improve vaccination rates, said PSA Queensland Branch State Manager Nicole Floyd MPS.
By ‘focusing on bringing the vaccine to the person rather than the person to the vaccine’, will improve vaccination rates, said PSA Queensland Branch State Manager Nicole Floyd MPS.
‘Instead of having a conversation with a consumer about vaccination and having to refer them on, pharmacists could vaccinate consumers there and then,’ she said. ‘Consumers will increasingly recognise pharmacists as demonstrated vaccine providers, and a convenient option to administer most vaccinations to the entire family.’
How did PSA’s advocacy help to bring about change?
The changes to Queensland’s vaccine legislation follow years of advocacy by PSA. This includes two submissions to the EPA consultation within a 12 month period requesting the removal of age barriers for pharmacist-administered vaccines, and allowing qualified pharmacists in all health care settings to vaccinate patients.
‘Pharmacists have been able to administer vaccines since 2014 after the Queensland Pharmacist Immunisation Pilot,’ said Ms Floyd. ‘Since then, it’s always been incremental changes to pharmacists’ authorisation based on the reactive need of Queenslanders.’
However, the COVID-19 pandemic demonstrated that pharmacists are a key vaccinating profession in all settings. ‘Pharmacists were administering COVID-19 vaccines in community pharmacies, in outreach and big public health clinics, and through the Royal Flying Doctor Service,’ said Ms Floyd.
‘Pharmacists have demonstrated their role, so now it’s about bringing the legislation up to [speed] to recognise that vital role.’
Queensland is one of the first jurisdictions to bring its local regulations in line with federal legislation allowing pharmacists to administer all vaccines under the National Immunisation Program (NIP) from 1 January 2024.
However, PSA’s Queensland Branch is hoping to address another key gap between the state and federal legislations, said Ms Floyd.
‘In the federal program, NIP funding for vaccines in community pharmacies is for patients aged 5 years and over,’ she said.
As it stands, there is a cost barrier for pharmacist-administered vaccines to any children under 5 years of age who are eligible for a NIP vaccine.
‘We’ve raised this with Minister Fentimen, who will escalate to the federal minister requesting that children under 5 years of age be included in the federal NIP program,’ Ms Floyd added.
How can pharmacists prepare for the changes?
To raise awareness of increased vaccine access among patients, pharmacists should take every opportunity to initiate proactive conversations about their vaccination status, said Ms Floyd.
‘The vaccine may not be administered that day,’ she said. ‘But by starting the conversation, you can work with patients to get their vaccinations up to date.’
Identifying patient need for vaccinations is key. ‘For example, influenza [vaccination rates] in pregnancy are quite low,’ said Ms Floyd. ‘So when expecting mums come into the pharmacy for pregnancy or baby-related products, initiate a conversation with them about their vaccination needs.’
Pharmacists can also discuss vaccinations for extended family members, such as whooping cough.
As pharmacists prepare to administer a range of new vaccines, the PSA Immunisation training resource hub can help to refresh their vaccination knowledge.
What other vaccination changes have occurred?
Last Friday (27 October 2023), the New South Wales Government also ‘cut unnecessary red tape’ to expand vaccination access across the state.
The vaccination changes in NSW include:
- Lowering of the age range that pharmacist immunisers can administer the Shingrix vaccine from 50 years and over to 18 years and over, in alignment with the eligible NIP age
- inclusion of monovalent meningococcal C vaccine for patients aged 5 years and over, allowing pharmacist immunisers to provide a full range of meningococcal vaccines to eligible cohorts
- removal of reference to the COVID-19 vaccine following the recent inclusion of the vaccine in the Australian Immunisation Handbook to be consistent with the approach for all other vaccines that pharmacist immunisers are authorised to immunise
- removal of the COVID-19 vaccine written consent requirements to align with consent requirements for all other vaccines i.e. that informed consent must be obtained, and documented evidence of verbal consent must be made.



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more



 Sponsorship information
Sponsorship information


 Talking to patients who have questions
Talking to patients who have questions




