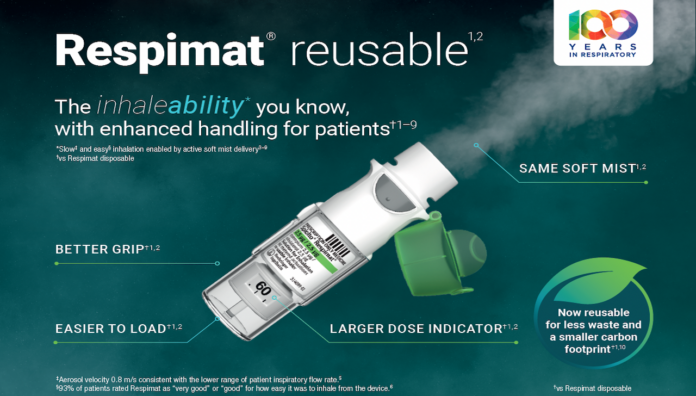
The inhaleability* you know, with enhanced handling for patients†1–9
*Slow‡ and easy§ inhalation enabled by active soft mist delivery.1–9
†vs Respimat disposable.
Same soft mist1,2
Allows patients to inhale slowly and gently3–5
Better grip†1,2
For enhanced everyday use
Larger dose indicator†1,2
Easy to see how many doses remain
Easier to load†1,2
Less force required to insert a cartridge
†vs Respimat disposable.

Learn more at www.respimat.com.au
HCP password: softmist
‡Aerosol velocity 0.8 m/s consistent with the lower range of patient inspiratory flow rate.5
§93% of patients rated Respimat as ‘very good’ or ‘good’ for how easy it was to inhale from the device.6

Please review Product Information before prescribing. Full Product Information is
available at www.boehringer-ingelheim.com.au/PI. Further Information is available
from Boehringer Ingelheim Pty Ltd.
References
- Dhand R et al. Int J COPD 2019;14:509–23.
- Wachtel H et al. Respir Drug Deliv 2020;1:195–204.
- SPIRIVA Respimat Consumer Medicine Information, March 2020.
- SPIOLTO Respimat Consumer Medicine Information, March 2020.
- Wachtel H et al. Pulm Ther 2017;3(1):19–30.
- Hodder R, Price D. Int J COPD 2009;4:381– 90.
- Schürmann W et al. Treat Respir Med 2005;4:53–61.
- Kardos P et al. Eur Respir J 2005;26(Suppl 49):338s.
- Dalby RN et al. Med Devices (Auck) 2011:4:145–55.
SPIRIVA®, SPIOLTO® and RESPIMAT® are registered trademarks of Boehringer Ingelheim. Boehringer Ingelheim Pty Ltd. ABN 52 000 452 308. 78 Waterloo Road, North Ryde, NSW 2113. PC-AU-102398. Date of preparation: November 2021. BICO23670W. Ward6


 Professor Margie Danchin[/caption]
Professor Margie Danchin[/caption]

 Dr Peter Tenni[/caption]
Dr Peter Tenni[/caption]
 How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]
How should we deprescribe gabapentinoids, according to the Maudsley Deprescribing Guidelines[/caption]



 Pharmacists have always prescribed, but they have the potential to prescribe much more
Pharmacists have always prescribed, but they have the potential to prescribe much more